Exit Seminar: Mechanism of Antibiotic Permeability Through the Gram-negative Bacterial Envelope
Abstract Title: The outer membrane of Gram-negative bacteria (GN) makes them distinct among superbugs that are associated with the development of antibiotic resistance. The outer membrane, and inner membrane, separated by the periplasm, form a double-membrane barrier to the entry of antibiotics into the cell. Several studies have been conducted to examine the role of outer membrane modifications such as porins, lipopolysaccharides, and efflux pumps on antibiotic resistance. However, there is a paucity of knowledge on how antibiotics behave in the periplasm, in order to gain access into their target region. My thesis focuses on understanding the mechanism of antibiotic permeability through the cellular envelope of Gram-negative bacteria. I studied the distribution of fluoroquinolones in the two aqueous compartments (periplasm and cytoplasm) of Escherichia coli using fluorescence intensity measurement and minimum inhibitory concentration (MIC) test. We treated the bacteria cells with each antibiotic, allowed the antibiotic to accumulate in the cells, fractionated the cells and quantified the concentration of accumulated antibiotic through the measurement of its fluorescence intensity. The compound accumulation assay showed that the efflux-deficient strain (?tolC) accumulated more antibiotic than the wild type (WT) strain, for all nine fluoroquinolones we tested. An analysis of the subfractions showed a greater accumulation of the antibiotic in the periplasm than in the cytoplasm. A positive correlation was seen between the MIC ratio (WT/?tolC) and the cytoplasmic accumulation ratio (?tolC/WT). This is an indication that the efficacy of a drug is a combined function of its ability to accumulate in the target region and inhibit the target. I also studied the impact of osmo-regulated periplasm glucans (OPGs) on antibiotic susceptibility in GN. We created E. coli and Salmonella typhimurium strains deficient in OPG production. We also created an E. coli strain that produced neutral OPGs. The drug susceptibility test showed that the strains that are either deficient in OPG production or produce neutral OPGs were less susceptible to the positively charged aminoglycosides compared to the WT strain. A similar response was observed when the bacteria strains were treated with the fluoroquinolones and tetracyclines. We speculate that this behavior is due to the net positive charge carried by these antibiotics from complexes formed with Mg2+. In contrast, the strains grew slower in the presence of a negatively charged cerufoxime. The observed MIC changes were not due to a leaky membrane. The OPG deficient strains produced significantly reduced amount of OPGs compared to the parent strain. Our study demonstrated that charge plays a significant role in OPG-mediated susceptibility to antibiotic. We also probed the role of OPGs on copper homeostasis in Gram-negative bacteria. We found that the disruption of the opgGH operon had an impact on the tolerance of GN bacteria to copper. Copper quenched the fluorescence of cytosolic GFP faster in the OPG- deficient strains compared to the WT strain. The GFP-quenching effect of copper ions was diminished with the increase of ionic strength, indicating that Cu2+ penetration into the cytoplasm slowed down under high salt condition. 





 Jonathan Rivnay
Jonathan Rivnay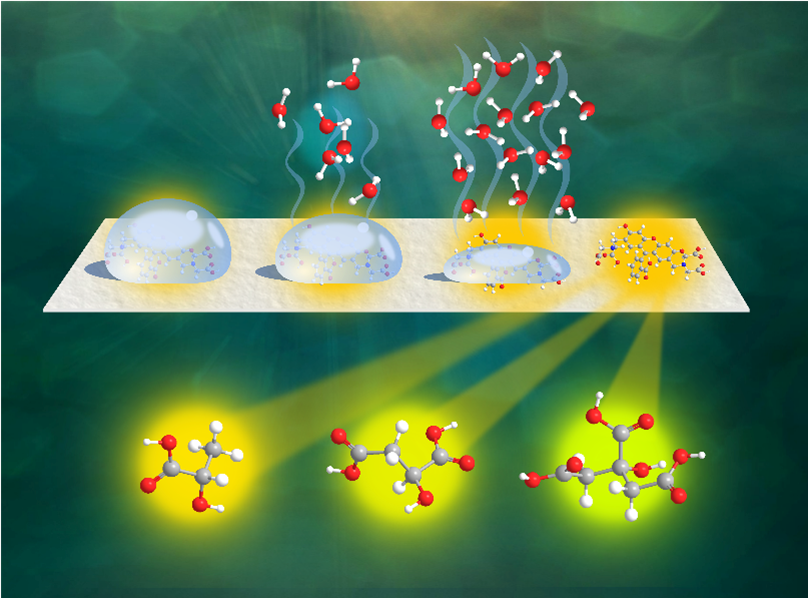 which are water solutions. However, aqueous media are very important in analytical, environmental, and biomedical applications, so it is valuable to adapt our supramolecular tools to them. With the right tools, even the weakest noncovalent interactions can be pressed into service in aqueous media. We have been using water-soluble polymers (e.g. dendrimers, hydrogels, conjugated polymers) as scaffolds to build multivalent supramolecular sensors that take advantage of the large number of interactions and of the preorganization of receptor sites afforded by such scaffolds, resulting in improved affinity in buffered aqueous solutions near neutral pH. We have successfully built systems for the detection of interesting guest families, including carboxylate anions, simple saccharides, heavy metal cations, and polycyclic aromatic hydrocarbons. These are examples of a general approach with two key advantages. On the one hand, installing known receptor chemistry on a polymer scaffold affords a modular approach to multivalency with minimal design and synthesis effort. This improves the apparent strength of weaker interactions and allows them to overcome desolvation costs in water. On the other hand, water-soluble macromolecular scaffolds impart solubility to water-incompatible receptor families.
which are water solutions. However, aqueous media are very important in analytical, environmental, and biomedical applications, so it is valuable to adapt our supramolecular tools to them. With the right tools, even the weakest noncovalent interactions can be pressed into service in aqueous media. We have been using water-soluble polymers (e.g. dendrimers, hydrogels, conjugated polymers) as scaffolds to build multivalent supramolecular sensors that take advantage of the large number of interactions and of the preorganization of receptor sites afforded by such scaffolds, resulting in improved affinity in buffered aqueous solutions near neutral pH. We have successfully built systems for the detection of interesting guest families, including carboxylate anions, simple saccharides, heavy metal cations, and polycyclic aromatic hydrocarbons. These are examples of a general approach with two key advantages. On the one hand, installing known receptor chemistry on a polymer scaffold affords a modular approach to multivalency with minimal design and synthesis effort. This improves the apparent strength of weaker interactions and allows them to overcome desolvation costs in water. On the other hand, water-soluble macromolecular scaffolds impart solubility to water-incompatible receptor families.