Exit Seminar: Inter and Intra Molecular Interactions to Control the Optoelectronic Properties of Materials

Functional materials used for optoelectronic applications are often employed in the solid-state regime. The properties of such solid-state materials are entirely dependent on the inter and intra molecular interactions that the molecules experience. Intermolecular interactions are interactions between two adjacent molecules and can be broken down into two subgroups: repulsive and attractive. Intramolecular interactions are interactions that occur within a molecule and include things like bonding, resonance, and electron distribution. These properties can be tuned through a number of techniques to afford desirable outcomes for various material applications. This dissertation will investigate how the tuning of the inter and intra molecular forces influence a material’s electronic and optical properties.

The dissertation will cover three projects that leverage control over hydrogen bonding, ionic interactions, and electron density to influence the optoelectronic properties of various systems. The first project attempted to increase intermolecular electronic couplings by using hydrogen bonded coproducts between an organic small molecule semiconductor and benzoic acids. Hydrogen bonding is a monodirectional interaction. The second project, in contrast, focuses on ionic interactions, which are multidirectional. These ionic interactions were investigated through the addition of a conjugated organic core to the inorganic anion in an organic inorganic hybrid material (OIHM) to improve material photoluminescence quantum yield (QY) efficiency. Additionally, alkyl substituents and anion size were changed to probe the effect of spacing on QY. In the third project of this dissertation, the focus moves from intermolecular interactions to intramolecular interactions. This project focuses on using electron donating and accepting groups to tune the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) levels of a metal complex to achieve more efficient deep red and near infrared (NIR) emission.
KEYWORDS: Intermolecular Interactions, Intramolecular Interactions, Optoelectronics, Organic Semiconductors, Light Emitting Materials

 Cerebrovasculature refers to the network of blood vessels in the brain, and its coupling with neurons plays a critical role in regulating ion exchange, molecule transport, nutrient and oxygen delivery, and waste removal in the brain. Abnormalities in cerebrovasculature and disruptions of the blood supply are associated with a variety of cerebrovascular and neurodegenerative disorders. Nanocarriers, a nano-sized drug delivery system synthesized from various materials, have been designed to encapsulate therapeutic agents and overcome delivery challenges in crossing the blood-brain barrier (BBB) to achieve targeted and enhanced therapy for these diseases. Unraveling the transport of drugs and nanocarriers in the cerebrovasculature is important for pharmacokinetic and hemodynamic studies but is challenging due to difficulties in detecting these particles within the circulatory system of a live animal. In this dissertation, we developed a technique to achieve real-time
Cerebrovasculature refers to the network of blood vessels in the brain, and its coupling with neurons plays a critical role in regulating ion exchange, molecule transport, nutrient and oxygen delivery, and waste removal in the brain. Abnormalities in cerebrovasculature and disruptions of the blood supply are associated with a variety of cerebrovascular and neurodegenerative disorders. Nanocarriers, a nano-sized drug delivery system synthesized from various materials, have been designed to encapsulate therapeutic agents and overcome delivery challenges in crossing the blood-brain barrier (BBB) to achieve targeted and enhanced therapy for these diseases. Unraveling the transport of drugs and nanocarriers in the cerebrovasculature is important for pharmacokinetic and hemodynamic studies but is challenging due to difficulties in detecting these particles within the circulatory system of a live animal. In this dissertation, we developed a technique to achieve real-time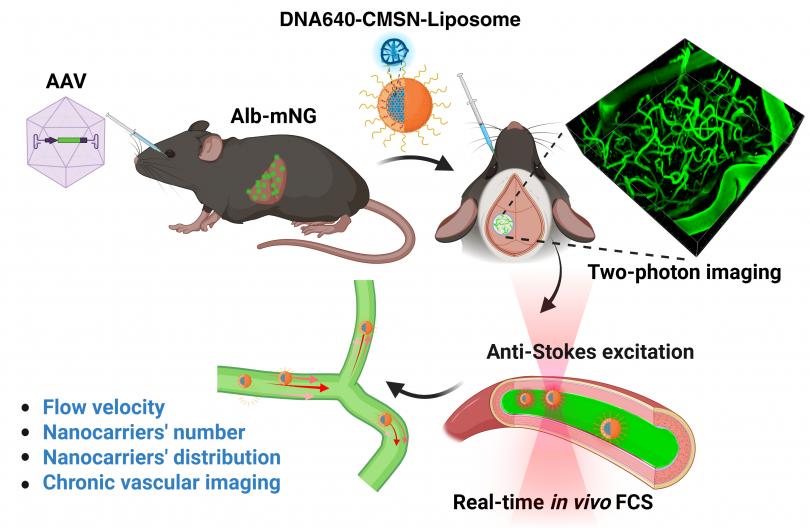
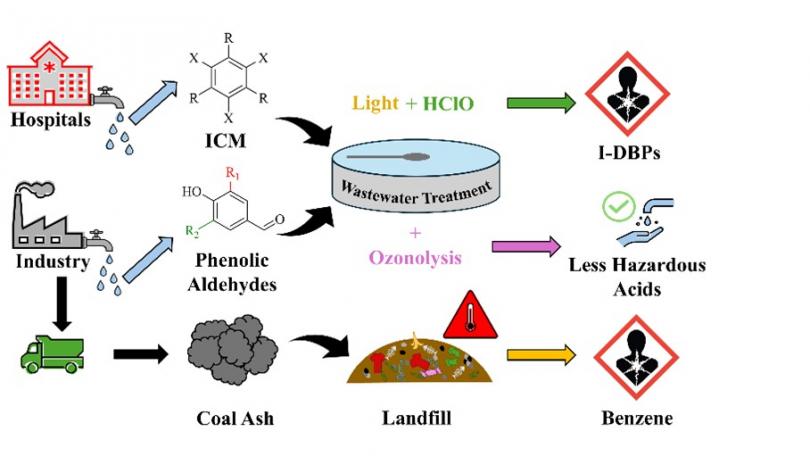
 The health of our communities depends on the effective treatment of both solid and liquid waste to eradicate hazardous pollutants before they can interact with living organisms or contaminate the environment. Daily, society generates solid waste (commonly destined for landfills) and liquid waste, (commonly discharged into wastewater systems) and without proper treatment, these wastes can release hazardous primary secondary pollutants. Industries producing wastewater with high pollutant concentrations, especially those utilizing lignin-based biomass, face complex challenges because each facility may require a tailored treatment approach. In response, this work investigates the use of ozonolysis to transform lignin monomers into smaller, less hazardous components that can be more efficiently managed by public wastewater systems. Furthermore, while conventional wastewater treatment systems are effective for common water quality issues, they can inadvertently allow complex compounds, such pollutants from hospital effluent, to pass through. Under simulated treatment conditions incorporating sunlight and chlorination, a pollutant released from medical facilities is degraded, but this process may also lead to the formation of carcinogenic disinfection by-products (DBPs) that pose direct toxicological risks to nearby communities. The implications extend to solid waste management as well. Chemical phenomena, such as those occurring in poorly understood elevated temperature landfills (ETLFs), can compromise treatment methods and increase community exposure to harmful pollutants. By monitoring hazardous components, such as volatile organic compounds (VOCs), over time, this work aims to elucidate the chemical reactions occurring both during treatment and in the environment thereafter. Ultimately, this research underscores the need for fundamental, innovative approaches to pollution transformation. Bridging the gap between existing practices for solid and liquid waste treatment will be critical to safeguarding environmental and public health.
The health of our communities depends on the effective treatment of both solid and liquid waste to eradicate hazardous pollutants before they can interact with living organisms or contaminate the environment. Daily, society generates solid waste (commonly destined for landfills) and liquid waste, (commonly discharged into wastewater systems) and without proper treatment, these wastes can release hazardous primary secondary pollutants. Industries producing wastewater with high pollutant concentrations, especially those utilizing lignin-based biomass, face complex challenges because each facility may require a tailored treatment approach. In response, this work investigates the use of ozonolysis to transform lignin monomers into smaller, less hazardous components that can be more efficiently managed by public wastewater systems. Furthermore, while conventional wastewater treatment systems are effective for common water quality issues, they can inadvertently allow complex compounds, such pollutants from hospital effluent, to pass through. Under simulated treatment conditions incorporating sunlight and chlorination, a pollutant released from medical facilities is degraded, but this process may also lead to the formation of carcinogenic disinfection by-products (DBPs) that pose direct toxicological risks to nearby communities. The implications extend to solid waste management as well. Chemical phenomena, such as those occurring in poorly understood elevated temperature landfills (ETLFs), can compromise treatment methods and increase community exposure to harmful pollutants. By monitoring hazardous components, such as volatile organic compounds (VOCs), over time, this work aims to elucidate the chemical reactions occurring both during treatment and in the environment thereafter. Ultimately, this research underscores the need for fundamental, innovative approaches to pollution transformation. Bridging the gap between existing practices for solid and liquid waste treatment will be critical to safeguarding environmental and public health.