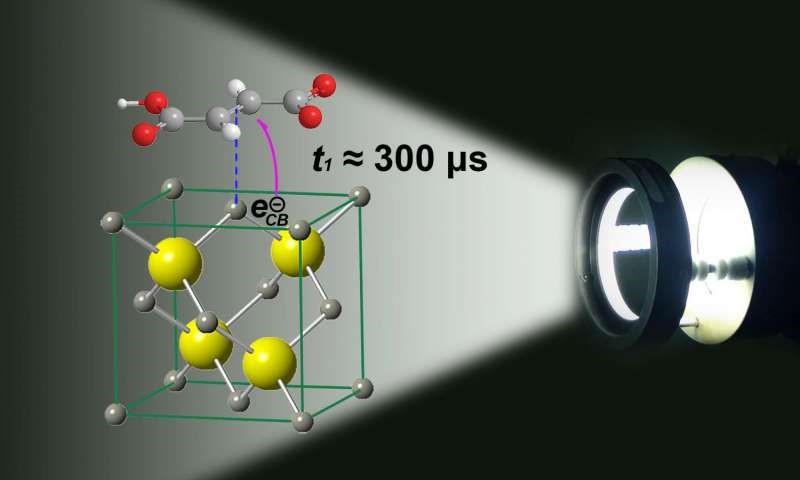
Finding the time scale for the effective transfer of electrons is not an easy task.
With the increasing need for renewable fuels scientists have attempted to harvest abundant sunlight while simultaneously reducing CO2. However, the process is generally inefficient and many aspects needed for improvement remain unknown. Chemists at the University of Kentucky have now contributed new knowledge to explain how sunlight energy is stored in chemical bonds creating energy rich molecules from depleted ones. The stable photocatalyst generates organic fuels with a rate of production that depends on the time spent on the surface by precursor molecules.
Finding the time scale for the effective transfer of reducing electrons in a photocatalyst capable of reducing species containing double bonds or CO2 is not an easy task. What is needed is a reducing electron that is generated upon excitation of a photocatalyst by the energy from sunlight. This reducing electron needs to be successfully transferred to the precursor molecule that yields the organic fuel. In laboratories, this has been achieved with several catalysts and modified materials; however, industrial implementation will require large yield improvements for this technology to be useful in the future.
Ruixin Zhou and Marcelo Guzman from the Environmental Chemistry Group at the University of Kentucky in Lexington, have developed a new approach to understand the complexity of the problem. The scientists have created a method to study the time scale that charge carriers remain active during illumination of a model zinc sulfide (ZnS) semiconductor suspended in water. As a result they have reported the time scale for effective electron transfer and valence band oxidation hole loss during photocatalysis.
Effective transfer of charge carriers
Upon excitation by light absorption of the photocatalyst charge carriers are produced, which can move to be transferred to an adsorbed molecule. Ultimately, the reducing electrons are transferred to the parent molecule (e.g., fumaric acid or CO2) to create a product that retains energy in chemical bonds.
For example, zinc sites on the surface of the nanomaterial interact weakly with the electrons in the double bond of a precursor molecule such a fumaric acid temporally fixing it for electrons to be transferred. The extent to which the parent molecules remain bound or adsorbed to the surface varies with the electrical potential and pH showing that surface charge plays an important role affecting the catalyst’s surface coverage. "The nanoparticles of the photocatalyst behave as microelectrodes on which the electrons are transferred from the excited semiconductor to the parent molecule, as was shown for the conversions of fumaric acid and CO2 to succinic and formic acids", says Ruixin Zhou, a Ph.D. candidate working in Guzman’s research group at the University of Kentucky. "And an electron donor is necessary to close the electrical circuit removing the accompanying positive charge created", Zhou explains. When the scientists add all the ingredients to an aqueous solution and shine light, the mixture starts producing the desired organic fuels.
The scientists managed to regulate the rate at which the material generated organic fuels by fine-modulating the time periods during which photons struck the surface. To do this, they varied how often the photons struck the photocatalyst’s surface, which is analogous to changing the break between balls being thrown by a tennis ball throwing machine. "We learned that the parent molecules are being transformed on the surface with a strong dependence on their reversible binding ability", Guzman says. The finding coincided with a theoretical framework explaining the contributions of reduction and oxidation half-reactions to the total current created by the charge carriers. "This is the first time ever that we are able to distinguish that the effective times needed to transfer electrons and holes on a semiconductor aimed to drive reduction reactions are quite long, even a few milliseconds", says Guzman.
In the future, the researchers want to apply these findings to study further the production of organic fuels and the degradation of pollutants. The goal has been to gain a better understanding of the photocatalytic mechanism governing these systems, which will contribute to improve the use of photocatalysis in applications such as CO2 conversion to hydrocarbons and wastewater treatment.
More information: Ruixin Zhou and Marcelo I. Guzman. Photocatalytic Reduction of Fumarate to Succinate on ZnS Mineral Surfaces, Journal of Physical Chemistry C (2016), 120, 7349, DOI: 10.1021/acs.jpcc.5b12380.